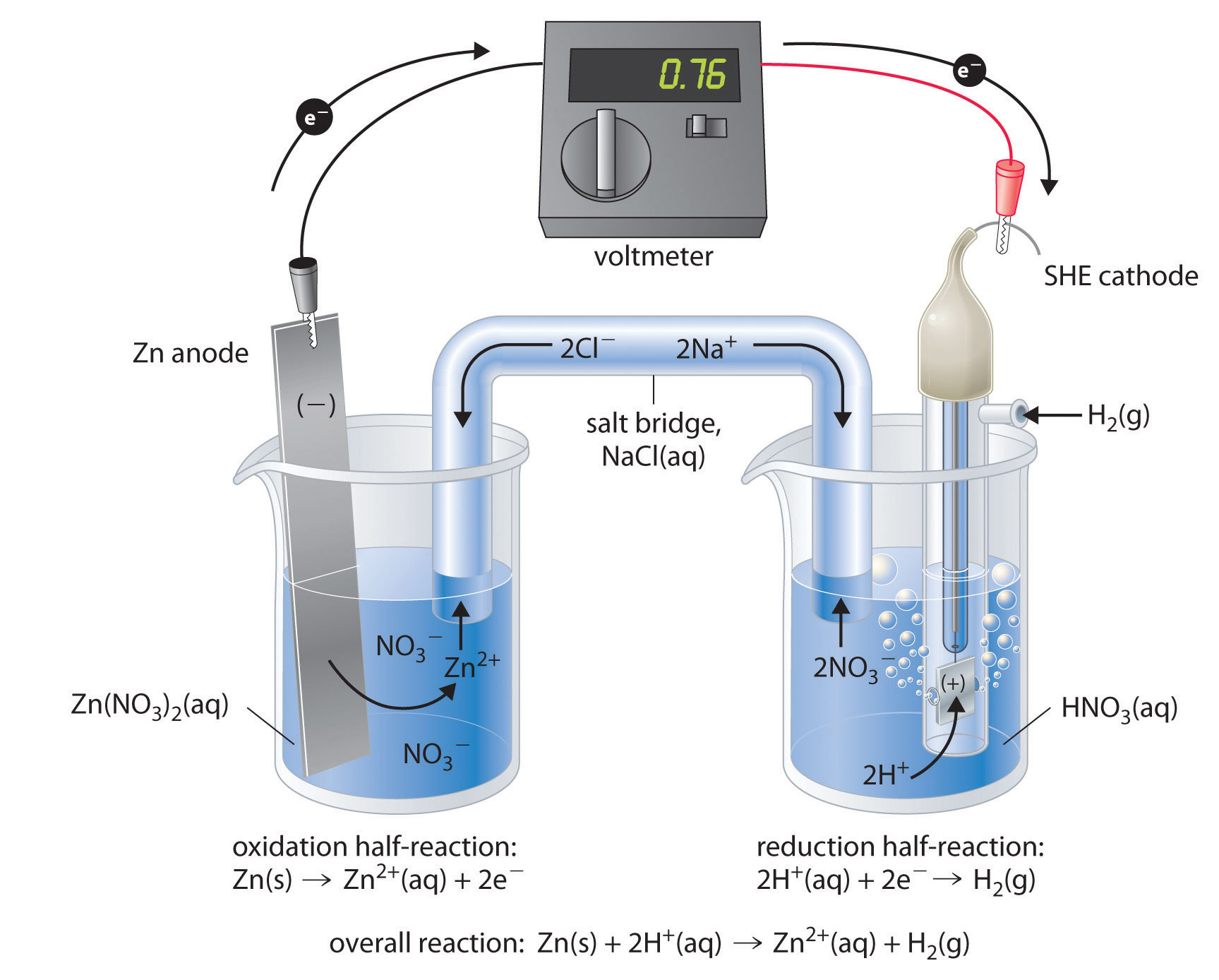
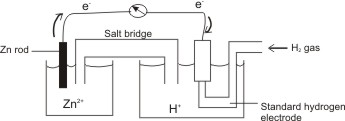
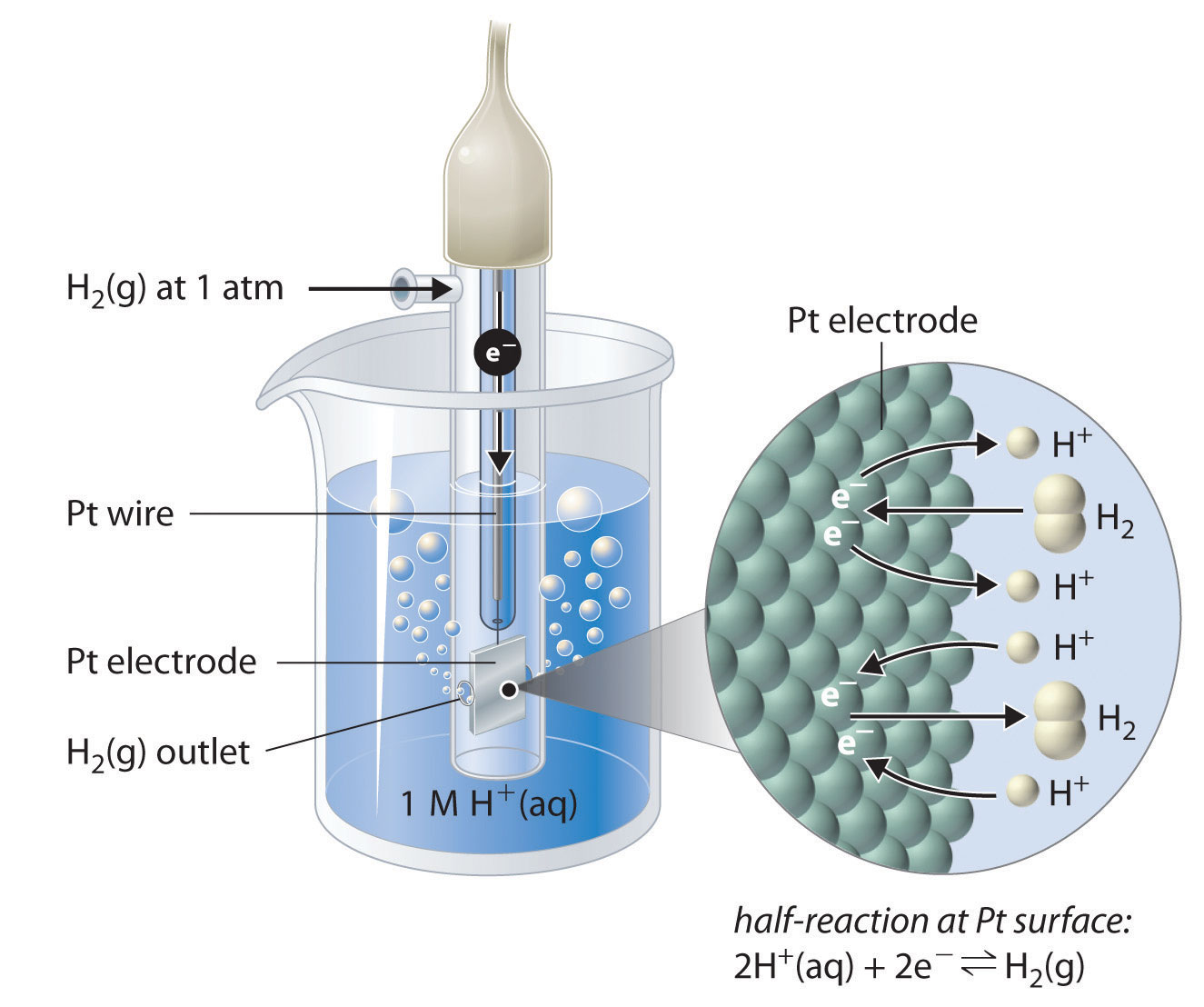
The standard electrode potential of Zn^2 + |Zn is - 0.76 V and that of Cu^2 + |Cu is 0.34 V . The emf (V) and the free energy change (kJmol^-1) ,

The standard reduction potentials of zinc, cadmium and copper are - 0.76 V, - 0.4 V and 0.34 V respectively. Select the correct statement.

Standard electrode potentials for the cathode and anode reactions in... | Download Scientific Diagram
How would you determine the reduction potential of Zn/Zn^2+ (aq)? - Sarthaks eConnect | Largest Online Education Community
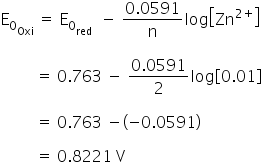
what is the potential of half cell consisting of electrode in solution at 250c e0oxi 0763v a 08221v b 8221v c 05282v d 9282v 5bbcsphh -Chemistry - TopperLearning.com

Soil and landscape factors influence geospatial variation in maize grain zinc concentration in Malawi | Scientific Reports
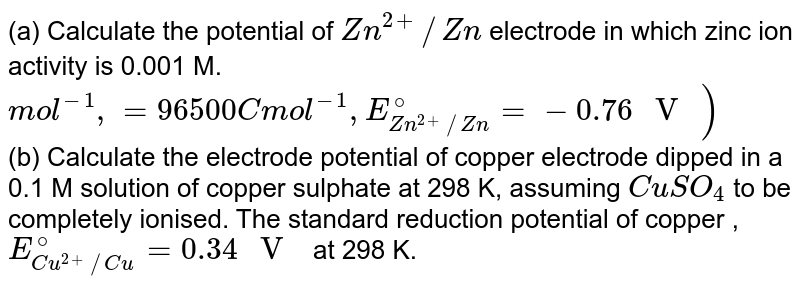
a) Calculate the potential of Zn^(2+)//Zn electrode in which zinc ion activity is 0.001 M. mol^(-1),=96500 C mol^(-1), E(Zn^(2+)//Zn)^(@)=-0.76" V ") (b) Calculate the electrode potential of copper electrode dipped in a
How to calculate the potential of zinc electrode capacity when in contact with 0.1M zinc sulphate solution in reference to hydrogen electrode when given the standard cell potential of Zn2 + /

The standard reduction potentials of zinc, cadmium and copper are - 0.76 V, - 0.4 V and 0.34 V respectively. Select the correct statement.